Specifications
| SKU | G468 |
| Unit quantity | 1.0 ml (10,000X) |
|
Description |
SafeView |
| SafeView Series | Post Staining Dye |
| LED Viewer Compatibility | Yes |
| Stain Color | Green |
| Applications | Safe Detection of dsDNA, ssDNA and RNA in agarose and polyacrylamide gels. |
| Note | All SafeView DNA Stains are ISO-13485 certified. |
| Dispose of SafeView DNA Stains as you would any other non-carcinogenic fluorescent dye (eg. Acridine orange; Propidium iodide). | |
| Shipping Conditions | Shipped on blue ice packs. |
| Storage Condition | Store at 4°C for up to 2 years. |
Documents
Supporting Protocol
MSDS
QC
Other
FAQs
- I want to know the differences of your products,Safe-Green,Safe-Red and Safe-white.
The main differences between Safe-Green, Safe-Red and Safe-white is their DNA binding dye and its excitation/emission spectra. Safe-Green has 500/522nm; Safe-White has approximate fluorescence excitation/emission maxima 358/461nm; Safe-Red has 488/562nm. Among these three, Safe-White has higher sensitivity than the other two colours.
- I have a question of usage for your Safe-White. As mentioned in your protocol,"Mix samples and DNA markers with a SafeViewTM dye at 1:5 dilution rate." means there is 50ul safe-white if the volume of my sample is 10ul?
If you have a 10ul sample, you should add 2ul of SafeView Dye to your sample before loading onto the gel.
- Can SafeView be a replacement for ethidium bromide? Can I do gel extraction with it?
SafeView can be used as a replacement for ethidium bromide as they both work on general agarose. We recommend using SafeGreen for downstream cloning applications as SafeView can interfere with the ligation reaction, yielding fewer colonies.
- How does SafeView work and why is it not carcinogenic?
There are fluorescent compounds in SafeView and these fluorescent compounds have the capability to bind to DNA. There may be some unknown effects of SafeView that have not been documented but that applies to the SYBR set as well; however, SafeView products are definitely not as carcinogenic as ethidium bromide.
- How do I use SafeView products?
The Safe-(Red, Green and White) loading dyes work the same as 6x loading dye, loaded with the sample. SafeView Classic is used directly in the gel and the running buffer.
- Does the SafeView differentiate double stranded nucleic acid and single stranded nucleic acid? Does the Safe-(Red, Green and White) work the same way?
Under UV, SafeView Classic emits a green fluorescence when bound to both single and double stranded DNA templates, therefore they cannot be differentiated by this method. It will emit a red fluorescence when bound to RNA templates. The SafeView Stains (Red, Green and White) do not perform in this way and will stain all nucleic acid templates one color.
- At what temperature do I store the SafeView products?
All the SafeView products should be stored at four degrees Celsius.
- Do I need a special filter for photography of DNA gels stained with SafeView?
Under UV light, SafeView Classic emits a green fluroescence when bound to both single and double stranded DNA templates. It will emit a red fluorescence when bound to RNA templates. No filter is necessary for viewing these colours, however a filter may be needed for photographing the gel.
- How long does the SafeView Classic stain last in a gel?
Our in-house testing has shown that SafeView stained gels (>10ng DNA loaded per lane) can still be effectively visualized up to 1 week later with only a slight decrease in brightness. Gels should be stored properly to maintain a good signal, at 4C in the dark, sealed in a plastic bag or pouch with wet paper towel loosely wrapped around the gel.
- Why is SafeView (G-108) stain not working on my samples?
Make sure you are following the protocol carefully. It is critical that both buffer and gel have SafeView dye in them otherwise it will not work. This is different from ethidium bromide. You can consider to add 2.5ul of SafeView (instead of 5ul) for every 100ml of running buffer, which will reduce background fluorescence and allow the bands to show with more contrast.
- Is it degradable, if so how fast and under what circumstances?
2 hours over 100C
- What is the sensitivity of the dyes?
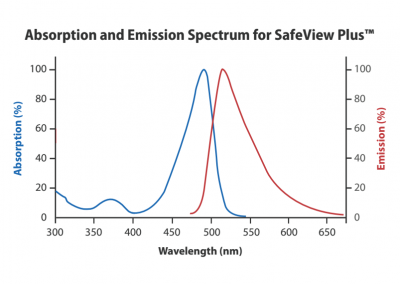
Safe-Green has Excitation Wavelength of 490nm and Emission Wavelength of 525nm, and its sensitivity range is between 0.2-0.6ng. Safe-Red has Excitation Wavelength of 540nm and Emission Wavelength of 630nm, and its sensitivity range is between 0.3-0.8ng. Safe-White has Excitation Wavelength of 370nm and Emission Wavelength of 470nm, and its sensitivity range is between 0.2-0.5ng. SafeView Classic emits green fluorescence when bound to dsDNA and ssDNA and red fluorescence when bound to RNA. This stain has one excitation (490 nm) and two emission spectra (520 nm and 635 nm) and the sensitivity range is between 0.1-0.3ng. SafeView Plus has Excitation Wavelength of 490nm and Emission Wavelength of 525nm and its sensitivity range is between 0.05-0.1ng.
- Can SafeView products be used post-stain?
Only SafeView Plus (G468) should be used in a post stain. SafeView classic and Safe Stains are not designed for post-staining. SafeView Classic must be added to the gel and the running buffer prior to the loading of the samples. Safe-(Red, Green and White) stains must be added to the sample before loading it to the gel.
- Why is the EtBr signal stronger in the pictures when I compare SafeView with EtBr?
A reason for this is that most gel doc systems have been optimized for EtBr so that is why the EtBr signal may be stronger in the pictures.
- I have a question regarding the protocol. If I were to use Safe-Pack. All I need to do is mix my DNA sample with the Safe-pack loading dye? There is no need for additional components such as mixing the agarose gel with safeview classic and as well as mixing the running buffer. There is only one step and that one step is mixing the DNA sample with your dye, is this correct?
Yes, it is correct. Safe-Pack (Red, Green and White) has different different protocol from Safeview Claasic. Safe-Pack requires only to be mixed with DNA sample before loading the gel. There is no need to add dye in the gel or buffer.
- What is the functional pH range for SafeGreen?
SafeGreen works best between pH 7 to 9.
- Is there any guidance on how long the gel lasts in the fridge once the stain has been added?
We know that gels with Safeview can last a week in the refrigerator. If it is stored longer than a week, the agarose gel will have resolution problem.
- Is there any guidance on how long the gel lasts in the fridge once the stain has been added?
We know that gels with Safeview can last a week in the refrigerator. If it is stored longer than a week, the agarose gel will have resolution problem.
- How do I dispose Agarose Gels stained with Safeview and how do I dispose buffers and gloves?
Dispose SafeView™ Classic as you would any other non-carcinogenic fluorescent dye (eg. Acridine orange; Propidium iodide). All gels and contaminated “non-sharp” lab debris (e.g., gloves, pads, towels, tubes, etc.) that are processed using this dye can be discarded in the trash. Spent running buffer solutions and destaining solutions that contain the dyes can either be collected and disposed of through the HWMU or collected and run through an approved filter device. The buffer solutions that have been run through the approved filter should be checked under the appropriate light source for complete removal of the dyes, and if it passes (does not fluoresce), the liquid can be disposed of down the drain with a copious amount of water as long as no other materials are present that would cause the material to be a Hazardous Waste. The filters that have been used up and are no longer effective must be disposed of through the HWMU.
- will I need an additional loading buffer for my samples?
The loading dye is included in the products. No additional loading buffer needed.
- Do SafeView products give problems in the process of cloning?
We recommend using Safe-Green (G108-G) for downstream cloning applications, as testing has shown it yields more colonies following the ligation reaction than SafeView (G108).
- What is the difference between Safe Green, Safe Red and Safe White? Does the loading buffer have different colors? Which filters do I have to use to detect light emission?
The difference is simply in colour of the dye, when viewed under UV the bands in the gel will show up in green, red or white.
- We see migrations and band shifting of our fragments. Are there any recommendations that you can give us to minimize this band shifting?
Shifting is unavoidable and quite natural for any fragments, regardless of the staining agent. We suggest to use SafeGreen ladders, which will give accurate molecular weight with no additional staining agents needed. http://www.abmgood.com/DNA-Ladder-Safe-Green™-100bp-Opti-DNA-Marker-Invitroge-G473.html http://www.abmgood.com/DNA-Ladder-Safe-Green™-1kb-Opti-DNA-Marker-Invitroge-G474.html
- We see shifting and migration of the DNA fragments. What are the recommendations to minimize this?
Shifting is unavoidable and quite natural for such fragments, regardless of the staining. We suggest using SafeGreen ladders, which will give accurate molecular weight with no additional staining agents needed. http://www.abmgood.com/DNA-Ladder-Safe-Green™-100bp-Opti-DNA-Marker-Invitroge-G473.html http://www.abmgood.com/DNA-Ladder-Safe-Green™-1kb-Opti-DNA-Marker-Invitroge-G474.html
- I cannot see 100bp and 200bp bands on a 1% gel. What should I do?
It is very difficult to detect 100bp and 200bp bands in 1% gel with any stains. Higher gel concentrations should be used, such as 2% agarose.
- Can I use SafeView products in Polyacrylamide gels?
Yes, we have tested our SafeView products for this application.
- What device do you use to see the electrophoresis bands?
We use both UV and LED transilluminators, our gel QC pictures in PCR department are taken with UV.
- Will staining appear the same when using UV light and LED (same colors)?
UV and LED will give the same colors.
- Which of the Safe stains will work with blue light / LED?
All of our SafeView stains have been tested in-house to be compatible with UV light. SafeView Classic, SafeView Plus, and SafeGreen will also work under blue light/LED. SafeRed and SafeWhite will only work under UV light. Safe View Plus should only appear green. SafeView Classic will be red for RNA and green for DNA.
- Can this be stored at -20°C?
It should be fine to store at -20°C, it will only prolong the shelf life (normally 2 years).
- Can this be stored at -20°C?
No, please store at 4°C only. Shelf life of 2 years.
- What is the concentration of G108?
20000X (5ul of the G108 in 100ml gel solution).
- Are SafeGreen stained DNA bands suitable for ligation purposes?
Yes
- Does the dye migrate in a 1% agarose gel?
Yes, the dye should migrate on a 1% gel.
- Does G108-G contain SDS?
No.
- What is the concentration of G108-G?
6X loading dye
- Would adding Safe-Green to the DNA ladder interfere with the migration rate of the loading dye of the ladder?
No, adding SafeGreen to the DNA ladder will not interfere with the DNA ladder's loading dye. You still need to add SafeGreen to the DNA ladder at the same ratio. To be more technically correct, SafeGreen, and all other EB alternative DNA binding dyes, would alter the migration pattern of DNA on agarose gel by shifting DNA to a larger molecular size; it is just simply because the overall molecular size of a particular DNA band increases as more dye is binding onto it. This is why we recommend mixing the DNA ladder and SafeGreen at the exact same 1:6 ratio, so everything on the gel will be subjected to the same concentration of SafeGreen and thus having the same shift.
- I'm a little bit confused about the light source that can be used for SafeRed. Below it is given, that the Ex/Em is 490/630nm, but in the last question (LED/UV light) it is given to use SafeRed with UV light source, normally in the range of 300nm. Is there a second excitation in the UV range?
Most labs are equipped with UV or LED light sources for viewing agarose gels, which is why we suggest these. As for SafeRed, yes, it does have an additional excitation wavelength of around 300nm. The other wavelength reported is for other people who may decide to use other light sources for the visualization.
- Can SafeView Classic penetrate cell membranes?
Yes, SafeView Classic can penetrate cell membranes.
- What is the size of the dye in SafeGreen after electrophoresis?
The dye should be at ~300bp after electrophoresis.
- After the electrophoresis runs, is it okay to recover the sample stained with Safe-red? Do I need to take special treatment to recover the sample DNA? I want to continue the cloning experiments with the sample after I check by electrophoresis.
Our Safe-Red™ (G108-R) will not affect the downstream cloning experiments, and thus no extra treatment is required to recover the stained sample DNA.
- Can Safe-Green penetrate the nucleus?
Yes, our Safe-Green™ stain indeed has the ability to penetrate the nucleus.
- Can this stain penetrate cell membranes?
Of our SafeView™ series DNA Stains, only SafeView™ (G108) and Safe-White™ (G108-W) can penetrate cell membranes, while the rest cannot.
- I used UV light for excitation, and my RNA is not glowing red as would be expected. Any idea why this is happening?
The complex interaction of SafeView Classic with DNA and RNA means that the emission spectrum is affected by secondary structure, temperature, ionic strength, pH and SafeView Classic concentration. Also, at high polynucleotides to SafeView Classic ratios, most nucleic acids will appear green. If the gels contain 6M urea at pH 3.5, all nucleic acids will appear green. So it is not unusual to observe the RNA bands as green under certain conditions.
- What color is the dye during electrophoresis?
The color of the dyes seen during migration would be blue, the same for all 3 dyes (Safe-Green/ Safe-Red/Safe-White). The size of the dye should be at approximately 300bp after electrophoresis. All three loading dyes have different excitation and emission wavelengths, so they will emit different colors. When bound to DNA, Safe-Green emits green fluorescence, Safe-Red emits red fluorescence, and Safe-White emits whitish pale blue fluorescence.
- Can you describe what particular imaging system that one should use to photograph the gel?
Any imaging system with UV and/or LED should work. No filter for Safeview is required, however a filter at ~500-650nm is optional.
References
- Dlusskaya, E. A., Atrazhev, A. M., & Ashbolt, N. J. “Colloid chemistry pitfall for flow cytometric enumeration of viruses in water” Water Research X 2:100025 (2019). DOI: 10.1016/j.wroa.2019.100025.
- Pereira, B. A., Zangeronimo, M. G., Castillo-Martín, M., Gadani, B., Chaves, B. R., Rodríguez-Gil, J. E., … Yeste, M. “Supplementing Maturation Medium With Insulin Growth Factor I and Vitrification-Warming Solutions With Reduced Glutathione Enhances Survival Rates and Development Ability of in vitro Matured Vitrified-Warmed Pig Oocytes” Frontiers in Physiology 9: (2019). DOI: 10.3389/fphys.2018.01894.