Antibody Hybridoma Sequencing Service
Finally obtained the desired hybridomas but do not know the antibody sequence that the cells produce? abm offers variable region sequencing from hybridomas. Choose one of our packages below to fulfill your requirements for Patent Application, Drug Development or Cell Line Authentication. Our sequencing services cover human, mouse and rabbit antibodies.
“We are very pleased with the outcome and turnaround time. Getting accurate antibody sequences from our hybridomas allowed us to move from secreted IgG to the recombinant expression of Fabs (antigen-binding fragment) for structure determination. The sequences recovered by the Applied Biological Materials Inc. team were of high quality, unambiguous, delivered according to a pre-established schedule, and resulted in us expressing high amounts of Fab that bound to our recombinant proteins with high affinity.”
Dr. Vincent Emond, Centre de recherche du CHU de Québec CHUL
Service Details
| SERVICE | DELIVERABLES | CAT. NO. | UNIT |
|---|---|---|---|
| Variable region sequencing | A detailed report on 5 sequencing reactions for light chain, and 5 sequencing reactions for heavy chain | C524 | 1 Clone |
| SERVICE | 1‑4 CLONES | 5‑9 CLONES | 10+ CLONES |
|---|---|---|---|
| Variable region sequencing | – | – | – |
Additional Info
Antibody Sequencing Advantages:
- Patent application
- Therapeutic antibody application for use in humanization
- Mass production
- Recombinant antibody generation (using single-chain antibody (scFv))
- Electronic banking (no need to maintain or store cells)
abm advantages:
- Experienced cloning and sequencing personnel
- Projects handle by PhD-level scientists
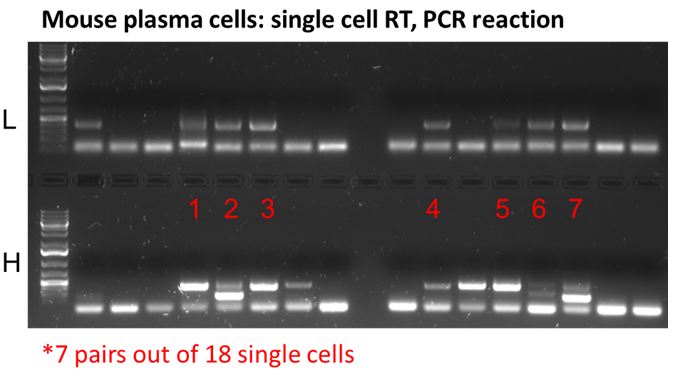
- Success at single cell level, please inquire for single cell sequencing
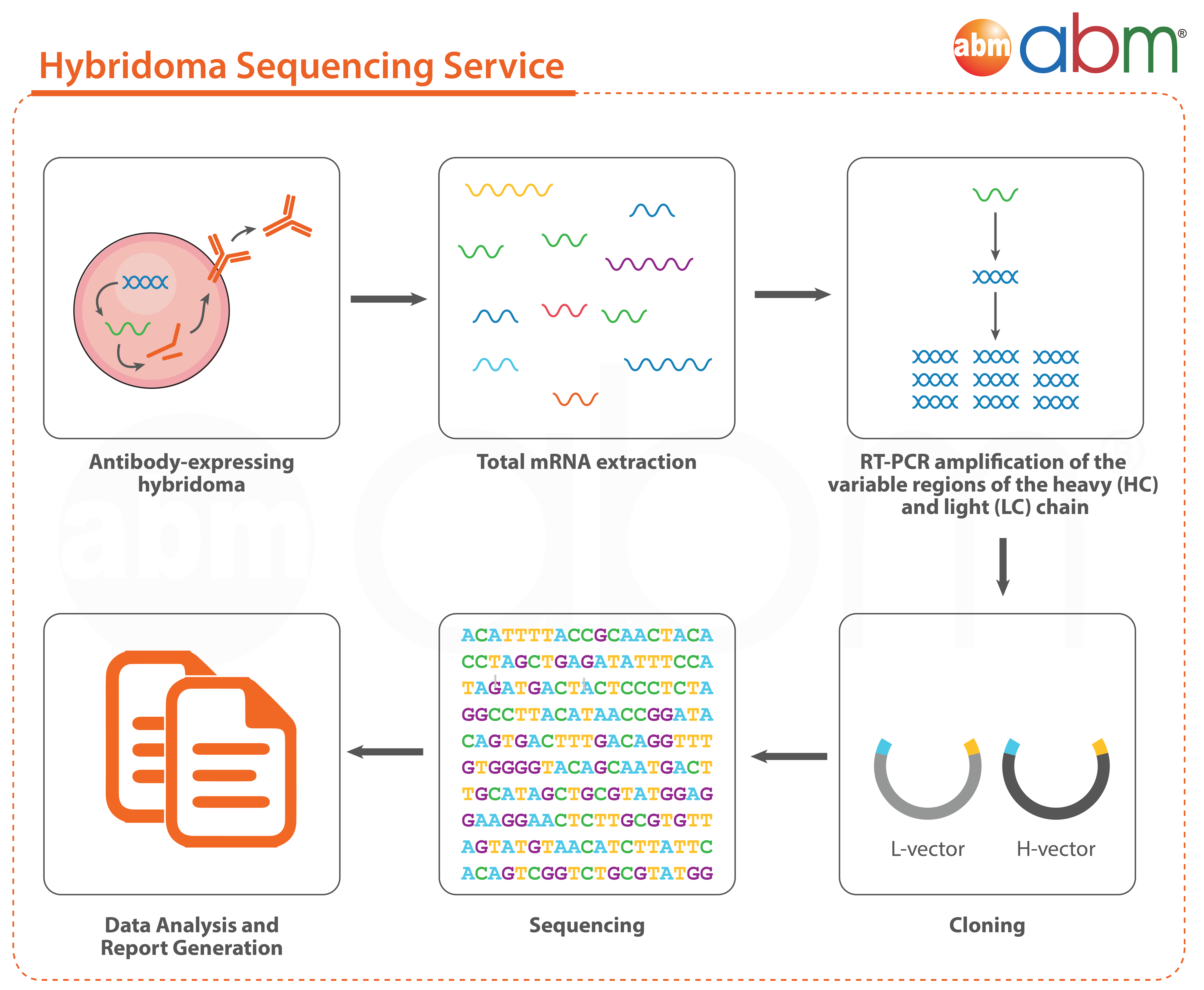
Workflow for variable region sequencing service (C524):
- RNA extraction from hybridoma cell line (if applicable)
- cDNA synthesis and amplification for light chain and heavy chain, separately
- Primer design
- Subcloning
- Bacterial culture and isolate plasmid from 5 colonies/sample, for both light chain and heavy chain
- Run 1 forward sequencing reaction per colony
Sample Submission Guideline:
| SAMPLE FORMAT | DETAILS |
|---|---|
| Total RNA |
|
| Frozen Cells |
|
Disclaimers and Policies
abm does not claim any rights on any antibody sequence. abm is not responsible for storage of the antibody sequence beyond three months. abm is not responsible for storage of any reagents associated with the customer (cell-line, RNA, PCR product and cloned plasmids) after one month of the report delivery.
A 30% non-refundable deposit is required to initiate the service. Our goal is always to deliver high-quality sequencing data to aid you in your research and downstream application. However, hybridoma sequencing by nature does not always deliver equally robust results due to a variety of factors, such as the quality of samples (low RNA content or high level of contaminates) that we receive. We will make every effort to deliver the sequencing results, although it is not possible to guarantee that it will always be achieved.
Documents
FAQs
Citations