Custom HSV Packaging Service
Several unique features of HSV-1 make vectors derived from this virus very appealing for preventive or therapeutic gene transfer. These include:
- Delivery of large transgenes to the nucleus of mammalian cells.
- Non-integrating, but can replicate separately from the host cell genome.
- The ability to establish life-long, non-toxic, latent infections in neurons; can establish stable transgene expression.
- Based on the HSV amplicon packaging method, which yields higher titers and has lower toxicity.
- All custom HSVs come with a GFP reporter for easy tracking of infection into target cells.
By pairing this technology with our expertise in virus packaging, abm offers custom HSV-1 Virus subcloning and virus production, allowing high-level gene expression in host cells.
Testimonial:
“If I have any special needs or concerns, they are always dealt with by the Technical Support Team. I have always been pleased with the product and will continue to use this service for my research.”
John Teem, Florida Department of Agriculture & Consumer Services
Additional Resources:
Service Details
Custom Baculovirus Plasmid Cloning
| SERVICE NAME | UNIT | CAT. NO. |
|---|---|---|
| Custom HSV Plasmid Cloning with Compatible Insert | 1 Service | C158 |
| Custom HSV Plasmid Cloning without Compatible Insert | 1 Service | C159 |
| Additional Charge for Extra 1kb*** | N/A | C100 |
Custom Cloning Service
| SERVICE NAME | DESCRIPTION | UNIT | CAT. NO. |
|---|---|---|---|
| Gene Synthesis | For any sequence up to 8kb in size Genes are fully-sequenced Must be ordered with Subcloning Service (C173) *minimum charge of $99.00 |
1.0 µg | C098 |
Custom HSV Production
| SERVICE NAME | UNIT | CAT. NO. |
|---|---|---|
| Custom HSV Production (106 IU/ml) | 10ml | C148 |
| Custom HSV Production (107 IU/ml) | 2ml | C149 |
HSV Controls
| SERVICE NAME | UNIT | CAT. NO. |
|---|---|---|
| HSV GFP Control Virus (106 IU/ml) | 10ml | C172 |
| HSV GFP Control Virus (107 IU/ml) | 2ml | C179 |
- ***Cloning of genes over 3kb are subject to an additional charge of $100.00/kb (C100). Please contact technical@abmgood.com for more information on available cloning vectors and restriction enzyme sites to qualify for the compatible insert service.
- C098: The above services must be ordered with a Custom HSV Production service, either C158 or C159, and a Custom HSV Cloning service, either C173 or C174.
- For customer-supplied vectors, the customer must indicate prior to the service if any reporter expression will be expected or not. The expected reporter expression, e.g. GFP, should not require induction of expression. Due to differences in excitation/emission wavelengths for different fluorophores, we may not be able to provide infection images for fluorescent reporter expression other than standard GFP/RFP/mCherry.
- The above services must be ordered with a Custom HSV Production service, either C158 or C159.
Additional Info
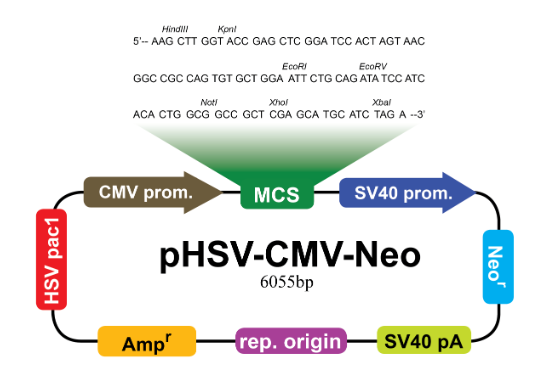
Herpes simplex virus (HSV) is a neurotropic herpes virus that can establish lifelong infections in its human host through latent maintenance in the ganglia of sensory neurons. Because of their wide host range, efficient infection, long-term persistence, capacity to accommodate large amounts of foreign DNA, and ability to deliver genes to post-mitotic cell types, HSV has showed considerable promise to establish long-term gene expression in most cells, particularly in neurons where other systems have low efficiency. Helper-free and amplicon HSV vectors have deletions in one or more essential viral genes and these can be used to express transgenes by replacing other non-essential viral genes.
Documents
FAQs
What is HSV amplicon packaging?
There are two major technologies for packaging HSV: the amplicon system and the helper-free system. The main differences between the two technologies are: (1) The amplicon method of packaging requires a helper virus, whereas the helper-free method does not; (2) the amplicon technology yields titers that are 3-4 logs higher than the helper-free technology; and (3) amplicon virus preparations show no toxicity, while helper-free preparations can be toxic. The notion that helper-free packaging is “better” than amplicon packaging is not supported in the literature. Contrary to popular belief, the helper virus is not neurotoxic, nor is replication-competent virus produced during the packaging procedure. Taking the above advantages into account, we employ the amplicon method instead of the helper-free method here at abm.
Is HSV toxic?
It has been reported that the HSV amplicon packaging procedure may produce virus that is neurotoxic. However, the improved purification and concentration steps developed by abm eliminates all potential toxicity from virus preparations. Some transgenes, on the other hand, can be slightly toxic if expressed at high levels. Therefore, it is suggested that the virus preparation be diluted between 1:1 and 1:3- with a solution of PBS, 10% sucrose and 25 mM HEPES at pH 7.3 if such toxicity is encountered.
How do you measure the virus titer?
We perform a plaque assay to determine the quantity of infectious virus.
Citations