
Germline-derived Pluripotent Stem Cells (gPS)
| CAT.NO | UNIT |
|---|---|
| T2027 | Vial |
| SKU | T2027 |
|---|---|
| Species | Mouse (M. musculus) |
| Species description | PND 35 Oct4-GFP Mice |
| Tissue/Organ/Organ System | Reproductive |
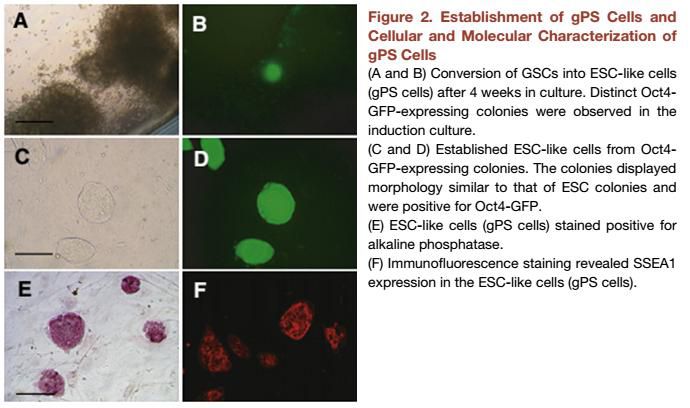
| Pluripotency | Positive for markers for alkaline phosphatase, and SSEA-1 by immunocytochemistry. It is capable of differentiating to cardiomyocytes or glial cells. |
| Caution | For Research Use Only, not for therapeutic or diagnostic purposes. |
| Cell Type | Ready-to-Use Stem Cells |
| Propagation Requirements | Cells are grown on Mouse Embryonic Fibroblast Feeder Cells (Cat. No. T2001). The basal medium for this cell line is Prigrow III medium available at abm, Cat. No. TM003. To make the completed growth medium, add the following components to the base medium to the stated final concentration: 15% fetal bovine serum (TM999), MEM nonessential amino acids (ThermoFisher; 11140-050), 2 mM L-glutamine (G275), 50 µM β-mercaptoethanol (CH045), 10 3 U/ml ESGRO, and Penicillin/Streptomycin (G255). Carbon dioxide (CO2): 5%, Temperature:37.0°C.. For in vitro glial differentiation: gPS cells aggregated to embryoid bodies. The base medium for this cell line is Prigrow III medium available from ABM (TM003). To make the completed growth medium, add the following components to the base medium to the stated final concentration: 15% fetal bovine serum (TM999), MEM nonessential amino acids (ThermoFisher; 11140-050), 2 mM L-glutamine (G275), 50 µM β-mercaptoethanol (CH045), 10 3 U/ml ESGRO (EMD Millipore; ESG1107), and Penicillin/Streptomycin (G255). Atmosphere: air: 95%, CO₂: 5%; Temperature: 37.0°C. Culture the cells with the complete growth media in presence of 10 ng/ml FGF2 (Z101455) and 20 ng/ml EGF (Z100135), followed by propagating cells in complete growth media with 10 ng/ml FGF2 (Z101455) and 10 ng/ml PDGF (Z100365) for 4 days. Differentiation is induced via 4-day growth factor withdrawal in presence of 30 ng/µl 3,3,5-tri-iodothyronine (T3; Sigma) and 200 µM ascorbic acid (AA; Sigma) in complete growth media. Atmosphere: air: 95%, CO₂: 5%; Temperature: 37.0°C. For in vitro cardiomyocytes differentiation: gPS cells cultivated in hanging drops as aggregates to embryoid bodies for 48 hours. The base medium for this cell line is Prigrow III medium available from ABM (TM003). To make the completed growth medium, add the following components to the base medium to the stated final concentration: 20% fetal bovine serum (TM999), MEM nonessential amino acids (ThermoFisher; 11140-050), 2 mM L-glutamine (G275), 50 µM β-mercaptoethanol (CH045), 10 3 U/ml ESGRO (EMD Millipore; ESG1107), and Penicillin/Streptomycin (G255). Atmosphere: air: 95%, CO₂: 5%; Temperature: 37.0°C. |
| Unit quantity | Vial |
Supporting Protocol
MSDS
QC
Other
What is the protocol for freezing iPSCs?
1) 4-5 days after cell split, when iPSCs reach a confluency of 30-60%, aspirate out spend medium and add 1ml 0.5mM EDTA/PBS. Incubate cells at 37C for ~5min. 2) When colonies start to detach, gently harvest all the cells into a 5-ml or 15-ml or 50-ml tube by pippeting 1-2 times. Avoid multiple pippetting. Breaking down clumps into single cells may substantially decrease cell survival. May add iPSC medium to harvest all the cells. 3) Spin down at 200g for 2-3 minutes. 4) Carefully aspirate out supernatent and gently resuspend cells in 0.5ml iPSC medium. 5) Add 0.5ml freezing medium (TM023) and ROCK inhibitor. Mix well by flipping the vial and transfer the medium to a cryovial. 6) Finally transfer the vial to -80C freezer for short-term storage (days to weeks). After cells are frozen, you may then transfer the vial to liquid nitrogen tank for long-term storage (years).
What is the recommended seeding density for iPSC?
IPSCs require seeder cells before thawing and plating the IPSC. Seed ~3x10^5 feeder cells in each well of a 6-well plate. After thawing the iPSCs, put all the cells from the vial into 1 well. After several days, you may start splitting the iPSCs into multiple wells depending on how many cells survived. The density mentioned above is for the seeder cells which should be at 3x10^5. Afterwards, please plate the entire IPSC into one well at 10^6 concentration.
- Igelmund, P et al. "Action potential propagation failures in long-term recordings from embryonic stem cell-derived cardiomyocytes in tissue cultur" Pflugers Arch 437(5):669-79 (1999). PubMed: 10087143.
- Brustle, O et al. "Embryonic stem cell-derived glial precursors: a source of myelinating transplants" Science 285(5428):754-6 (1999). PubMed: 10427001.
- Ko, K et al. "Induction of pluripotency in adult unipotent germline stem cells" Cell Stem Cell 5(1):87-96 (2009). DOI: 10.1016.
- Maltsev, VA et al. "Embryonic stem cells differentiate in vitro into cardiomyocytes representing sinusnodal, atrial and ventricular cell types" Mech Dev 44(1):41-50 (1993). PubMed: 8155574.


